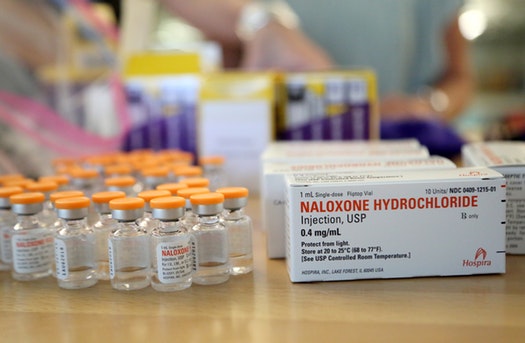
Naloxone HCL 10 vials
$250.00
-
-
Naloxone Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of naloxone hydrochloride in water for injection. Each milliliter (mL) contains 0.4 mg naloxone hydrochloride and sodium …
- CLINICAL PHARMACOLOGY
Complete or Partial Reversal of Opioid Depression – Naloxone prevents or reverses the effects of opioids including respiratory depression, sedation and hypotension. Also, Naloxone can reverse the …
- PHARMACOKINETICS
Distribution – Following parenteral administration naloxone is rapidly distributed in the body and readily crosses the placenta. Plasma protein binding occurs but is relatively weak. Plasma …
- INDICATIONS AND USAGE
Naloxone Hydrochloride Injection is indicated for the complete or partial reversal of opioid depression, including respiratory depression, induced by natural and synthetic opioids including …
- CONTRAINDICATIONS
Naloxone hydrochloride injection is contraindicated in patients known to be hypersensitive to naloxone hydrochloride or to any of the other ingredients contained in the formulation.
- WARNINGS
Drug Dependence – Naloxone hydrochloride injection should be administered cautiously to persons, including newborns of mothers, who are known or suspected to be physically dependent on opioids. In …
- PRECAUTIONS
General – In addition to naloxone, other resuscitative measures such as maintenance of a free airway, artificial ventilation, cardiac massage, and vasopressor agents should be available and …
- ADVERSE REACTIONS
Postoperative – The following adverse events have been associated with the use of naloxone hydrochloride injection in postoperative patients: hypotension, hypertension, ventricular tachycardia and …
- DRUG ABUSE AND DEPENDENCE
Naloxone hydrochloride injection is an opioid antagonist. Physical dependence associated with the use of naloxone hydrochloride injection has not been reported. Tolerance to the opioid antagonist …
- OVERDOSAGE
There is limited clinical experience with naloxone hydrochloride injection overdosage in humans. Adult Patients – In one small study, volunteers who received 24 mg/70 kg did not demonstrate …
- DOSAGE AND ADMINISTRATION
Naloxone Hydrochloride Injection, USP may be administered intravenously, intramuscularly, or subcutaneously. The most rapid onset of action is achieved by intravenous administration and it is …
- HOW SUPPLIED
Naloxone Hydrochloride Injection, USP is supplied in the following: Unit of SaleConcentration – NDC 0409-1215-01 – Carton of 10 – Single-dose Fliptop Vials – 0.4 mg/1 mL (0.4 …
- PRINCIPAL DISPLAY PANEL – 1 mL Vial Label
NDC 0409-1215-21 – 1 mL Single-dose – NALOXONE HCl – Injection, USP 0.4 mg/mL – For I.V., I.M., or S.C. use. Protect from light. Rx only – Hospira – Distributed by – Hospira, Inc., Lake Forest, IL 60045 …
- PRINCIPAL DISPLAY PANEL – 1 mL Vial Carton
1 mL Single-dose Fliptop Vial – NDC 0409-1215-01 – Contains 10 of NDC 0409-1215-21 – NALOXONE HYDROCHLORIDE – Injection, USP – Rx only – 0.4 mg/mL – Protect from light. For Intravenous, Intramuscular, or …
- PRINCIPAL DISPLAY PANEL – 10 mL Vial Label
10 mL Multiple-dose – NALOXONE HCl – Injection, USP – 4 mg/10 mL (0.4 mg/mL) Protect from light. For I.V., I.M. or S.C. use. Dist. by Hospira, Inc., Lake Forest, IL 60045 USA
- PRINCIPAL DISPLAY PANEL – 10 mL Vial Carton
One Unit/NDC 0409-1219-41 – 10 mL Multiple-dose Fliptop Vial – NALOXONE – HYDROCHLORIDE – Injection, USP – 4 mg/10 mL – (0.4 mg/mL) Protect from light. Rx only – Hospira
-
- INGREDIENTS AND APPEARANCE
Product Information






Reviews
There are no reviews yet.