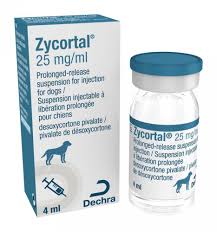
Buy Zycortal
Buy Zycortal is a veterinary medicine used to treat dogs with Addison’s disease. Addison’s disease is a condition, known as hypoadrenocorticism, where the adrenal glands (located above the kidneys) do not produce enough of two steroid hormones, called cortisol and aldosterone. The lack of aldosterone can cause fluid loss, dehydration and weight loss.
this product is used long-term to replace the missing aldosterone. A corticosteroid medicine is likely to be needed in addition, to replace the cortisol.
Zycortal contains the active substance desoxycortone pivalate.
This page contains information on Suspension for veterinary use.
The information provided typically includes the following:
Zycortal Suspension Indications
Warnings and cautions for Zycortal Suspension
Direction and dosage information for Zycortal Suspension
Zycortal Suspension
This treatment applies to the following species:
Dogs
Company: Dechra
Desoxycortone pivalate prolonged-release suspension for injection
25 mg/mL
STERILE
Veterinary Use Only
DIN 02453657
THERAPEUTIC CLASSIFICATION:
Mineralocorticoid.
Description
Chemically, desoxycorticosterone pivalate is:
21-(2,2-dimethyl-1-oxopropoxy)-pregn-4-ene-3,20-dione.
The structural formula is:
Molecular formula: C26H38O4
Active Ingredients
Each mL contains 25 mg desoxycortone pivalate, USP (equivalent to 19.9 mg/mL desoxycortone as base).
Preservative: Chlorocresol, 1 mg/mL.
Zycortal Suspension Indications
For use as replacement therapy for mineralocorticoid deficiency in dogs with primary hypoadrenocorticism (Addison’s disease).
Dosage and Administration
Prior to each use, thoroughly shake the vial to resuspend the product. Zycortal Suspension replaces the mineralocorticoid hormones only. Dogs with combined glucocorticoid and mineralocorticoid deficiency should also be treated with an appropriate glucocorticoid.
this Suspension is intended for long term administration at intervals and dosages dependent upon individual patient response. Tailor the dose of Zycortal Suspension and the concurrently administered glucocorticoid replacement therapy to the individual animal based on clinical response and normalization of Na+ and K+ concentrations.
Initial dose: The initial dose is 2.2 mg/kg body weight administered by subcutaneous injection.
Interim monitoring visit: Re-evaluate the dog and measure the serum sodium/potassium (Na+/K+ ratio) approximately 10 days after the first dose, which is the time to maximum concentration (Tmax) of desoxycorticosterone (see CLINICAL PHARMACOLOGY). If the dog’s clinical signs have worsened or not resolved, adjust the dose of glucocorticoid and/or investigate other causes of the clinical signs.
Be the first to review “Zycortal” Cancel reply
Related products
ORAL
ORAL
ORAL
ORAL






Reviews
There are no reviews yet.